

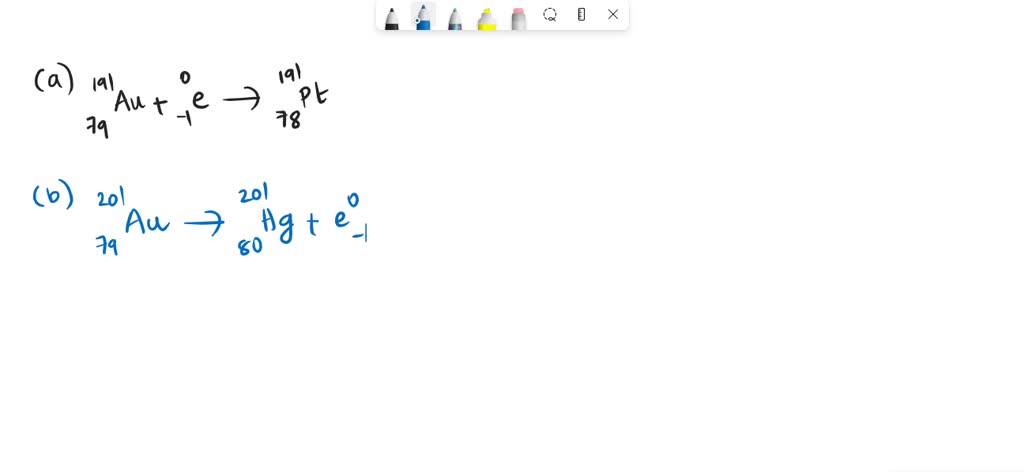
(credit a: modification of work by Jens Maus)įor example, F-18 is produced by proton bombardment of 18O and incorporated into a glucose analog called fludeoxyglucose (FDG). The scans it produces can be used to image a healthy brain (b) or can be used for diagnosing medical conditions such as Alzheimer’s disease (c). (Figure) summarizes these types of decay, along with their equations and changes in atomic and mass numbers.Ī PET scanner (a) uses radiation to provide an image of how part of a patient’s body functions. The choice is primarily due to kinetic factors, with the one requiring the smaller activation energy being the one more likely to occur. Whether electron capture or positron emission occurs is difficult to predict. This increases the n:p ratio, and the daughter nuclide lies closer to the band of stability than did the parent nuclide. Electron capture has the same effect on the nucleus as does positron emission: The atomic number is decreased by one and the mass number does not change. Like positron emission, electron capture occurs for “proton-rich” nuclei that lie below the band of stability. In most cases, the energy emitted will be in the form of an X-ray. As the outer electron drops into the vacancy, it will emit energy. The loss of an inner shell electron leaves a vacancy that will be filled by one of the outer electrons. The radiation produced during radioactive decay is such that the daughter nuclide lies closer to the band of stability than the parent nuclide, so the location of a nuclide relative to the band of stability can serve as a guide to the kind of decay it will undergo ( (Figure)).Įlectron capture occurs when an inner shell electron combines with a proton and is converted into a neutron. The daughter nuclide may be stable, or it may decay itself. The unstable nuclide is called the parent nuclide the nuclide that results from the decay is known as the daughter nuclide. The spontaneous change of an unstable nuclide into another is radioactive decay. During the beginning of the twentieth century, many radioactive substances were discovered, the properties of radiation were investigated and quantified, and a solid understanding of radiation and nuclear decay was developed. Among them were Marie Curie (the first woman to win a Nobel Prize, and the only person to win two Nobel Prizes in different sciences-chemistry and physics), who was the first to coin the term “radioactivity,” and Ernest Rutherford (of gold foil experiment fame), who investigated and named three of the most common types of radiation. Describe common radiometric dating techniquesįollowing the somewhat serendipitous discovery of radioactivity by Becquerel, many prominent scientists began to investigate this new, intriguing phenomenon.Calculate kinetic parameters for decay processes, including half-life.Write and balance nuclear decay equations.

Identify common particles and energies involved in nuclear decay reactions.Recognize common modes of radioactive decay.By the end of this section, you will be able to:


 0 kommentar(er)
0 kommentar(er)
